Which One of the Following Systems Has the Highest Entropy
One can measure ATP production from isolated mitochondria in vitro in a test tube. Which of the following has the highest entropy S.
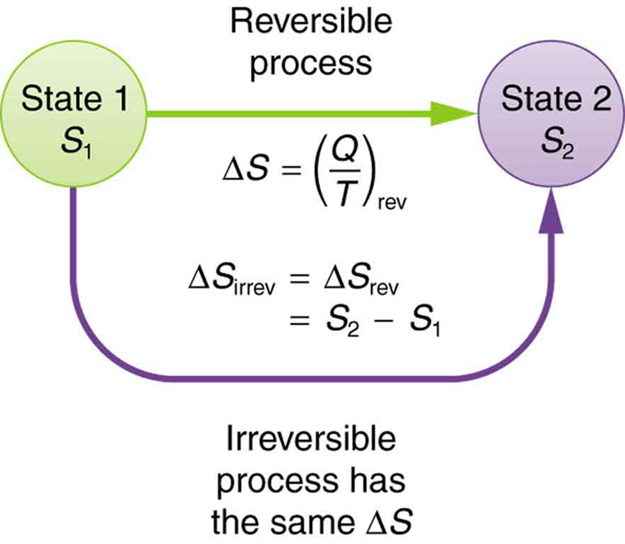
What Is Entropy Definition Equation And Formulas Of Entropy
The entropy by definition is the degree of randomness or disorder in matter.
. One of the better ones that should be considered is the one in the following link because it is free to download. There are such systems on the market for phone management systems. 10mL of water at 50C c.
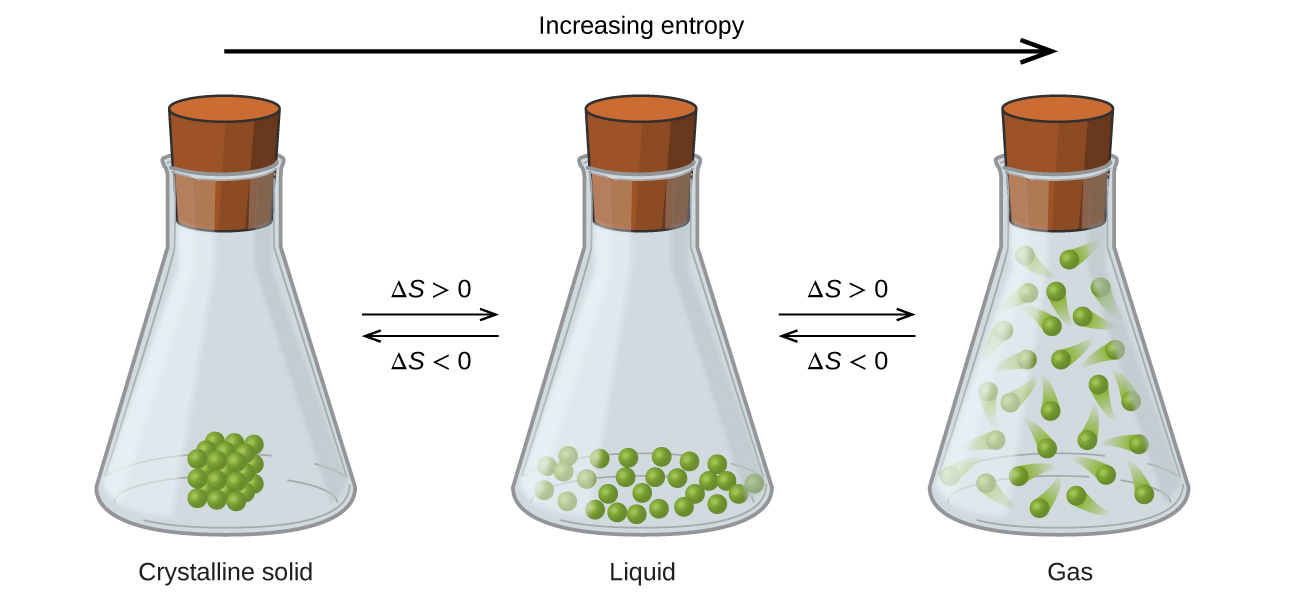
C Absolute Entropy d Absolute Free Energy. Gas particles have higher kinetic energy than liquid or solid particles and they have more space available for them to move in a faster and more random way than is liquid or solid thus the entropy S is in the following orderS_Gas S_Liquid S_Solid. 2 kg of HCN d.
CH 3CH 2OH e. Enthalpy Free energy Work Entropy. One of the formulations of the second law of thermodynamics.
In which of these systems is the entropy decreasing. If we look at the three states of matter. Thus The entropy of an isolated system never decreases.
Salt dissolving in water D. Which of the following systems has the highest entropy. 10 mL of water at 100 C d.
Which one of the following systems has the highest entropy. H_2 g HBrO_4 g HBr g He g The SI units are Joules J for each of the following EXCEPT ___. Which of the following would have the highest entropy assuming one mole of each substance and the same temperature for each.
This is the best answer based on feedback and ratings. We view proof that the world tends toward highest possible entropy many type of locations in our stays. Air escaping from a tire B.
Thus systems that are disorganized have greater entropy. C Absolute Entropy Explanation. Which one of the following systems has the highest entropy.
10 mL of water at 100C 10 mL of water at 50C 10 mL of water at 10. 05 g of HCN b. It will be maximum in gases followed by liquids and least in solids.
The second law of thermodynamics states that entropy in a closed system will continuously increase until the entropy reaches the maximum level at equilibrium. 10 mL of water at 50 C c. A gas condensing to a liquid Weegy.
THIS SET IS OFTEN IN FOLDERS WITH. Once the system reaches the macropartition with the highest multiplicity highest entropy it will stay there. The solid wood burns and becomes ash smoke and gases all of which spreview energy outwards more quickly than the solid fuel.
The Third Law of Thermodynamics is concerned with the limiting behavior of systems as the temperature approaches absolute zero. The Meaning of Oxidation and Reduction. Here hydrogen gas has more entropy as it shows more randomnessdisorderliness due to less molar mass than all the given substances and also in the gas phase.
A cup of water at 50 degrees c c. Which one of the following systems has the highest entropy. The entropy of the universe increases with any energy change that.
1 mol of HCN c. Which of the following has the highest entropy S. A cup of ice at 15 degrees c 2 the change in free energy or δg represents the maximum amount of energy available to do useful work.
A cup of ice at 0 degrees c a cup of water at 50 degrees c a cup of water at 0 degrees c a cup of ice at 15 degrees c which of the following systems has the lowest entropy. 88 which one of the following systems has the highest. Changing the pH of the solution that the mitochondria are in results in a similar change the pH of the intermembrane space.
10mL of water at 10C b. A campfire is an instance of entropy. Solid Liquid and Gas we can see that the gas particles move freely and therefore the degree of randomness is the highest.
Which of the following has the highest entropy S. Of particlestherefore here 10ml of water at 100C has highest entropy. Entropy is a measure of chaos or disorder in a system.
Most thermodynamics calculations use only entropy differences so the zero point of the entropy scale is often not important. See the answer See the answer See the answer done loading. Hence here the gas is only hydrogen and it has the highest entropy.
Hence option B is correct. 10mL of water at 100C d. All have the same entropy because all our water.
Greater the randomness of molecules of a substance greater is the entropy. Indicate which of the following has the highest entropy at 298 K. All have the same entropy because all are water.
In which of these systems is the entropy decreasingA. If a system is left to change spontaneously in what state will it end. A cup of water at 0 degrees c d.
2 mol of HCN e. Which of the following systems has the highest entropy. 1 Answer entropy is directly proportional to the temperature of the substance and the no.
G AS L iq u id. 10 mL of water at 100C 10 mL of water at 50C 10 mL of water at 10C. Entropy by definition is the degree of randomness in a system.
Entropy is defined as the amount of disorder within a system. All have the same entropy because all are water. This problem has been solved.
All of the above have the same entropy at 298 K. A cup of ice at 0 degrees c b. Which systems has the highest entropy.
For the second question the system with the lowest entropy is sugar crystals in 95 degrees centigrade cup of coffee. Correct option is B The measure of randomness of a substance is called entropy. Indicate which of the following has the lowest standard molar entropy S.
Which of the following has the highest entropy. For liquid state still the particles are moving but less freely than the gas particles and more than the solid particles. Entropy is a measure of disorder.
Find an answer to your question Which one of the following systems has the highest entropy. Sugar crystals in a 95c cup of coffee sugar crystals in a 27c cup of coffee sugar crystals in a 53c. Entropy to macropartitions with higher multiplicity higher probability higher entropy.
T T T 1. Enthalpy Free energy Work Entropy. 10 mL of water at 10 C b.
For the first one the system with the highest entropy is a cup of water at 0 degress centigrade. Which one of the following systems has the highest entropy.

Entropy And Free Energy Ppt Video Online Download

Entropy Transfer An Overview Sciencedirect Topics

Entropy Spontaneous Reactions Many Spontaneous Chemical Reaction Are Exothermic E G Burning Methane To Produce Carbon Dioxide And Water Some Endothermic Ppt Download

Entropy Transfer An Overview Sciencedirect Topics

Pin By Kundan Kumar On Space And Astronomy Astronomy Terms Thermodynamics Physics

Phy 212 General Physics Ii Chapter 20 Entropy Heat Engines Lecture Notes Ppt Download
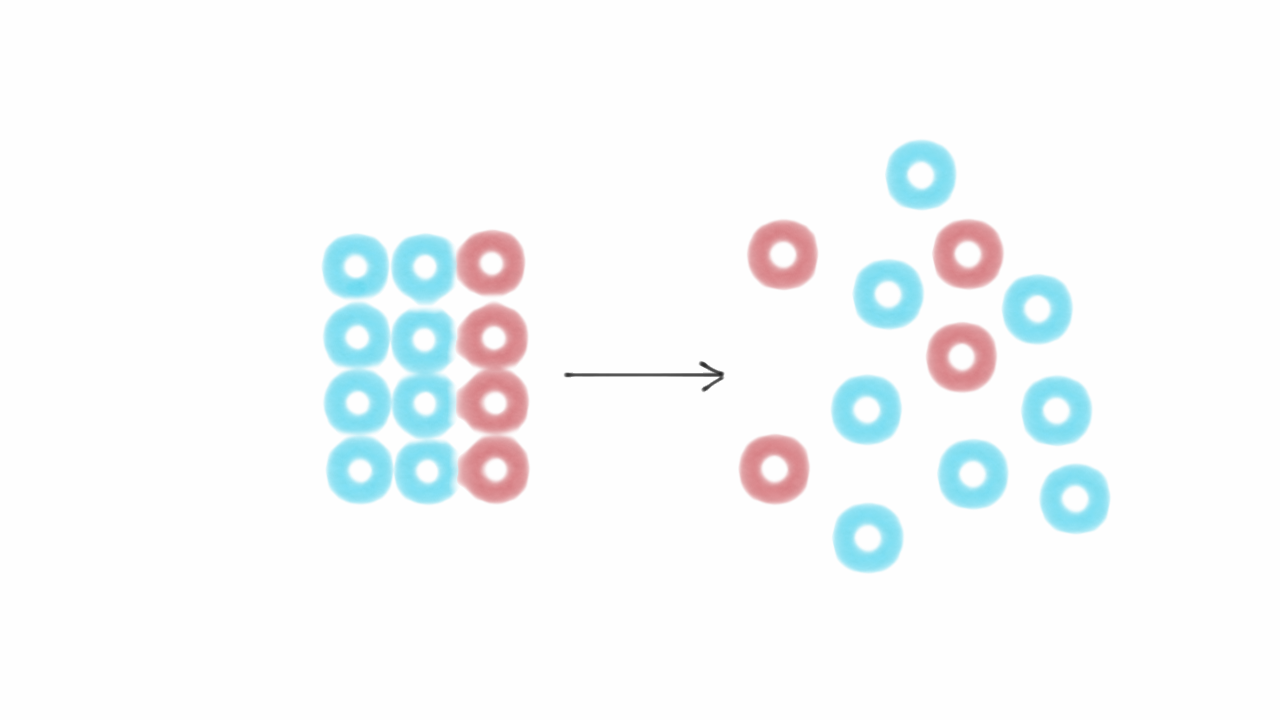
Entropy The Hidden Force That Complicates Life Farnam Street

Pin On 5e Gunslinger Character Options

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

Thermodynamics Doodle Graphic Thermodynamics Physical Chemistry Chemistry

What Is Entropy Definition Equation And Formulas Of Entropy

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

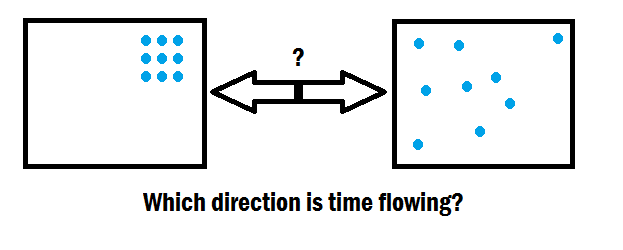
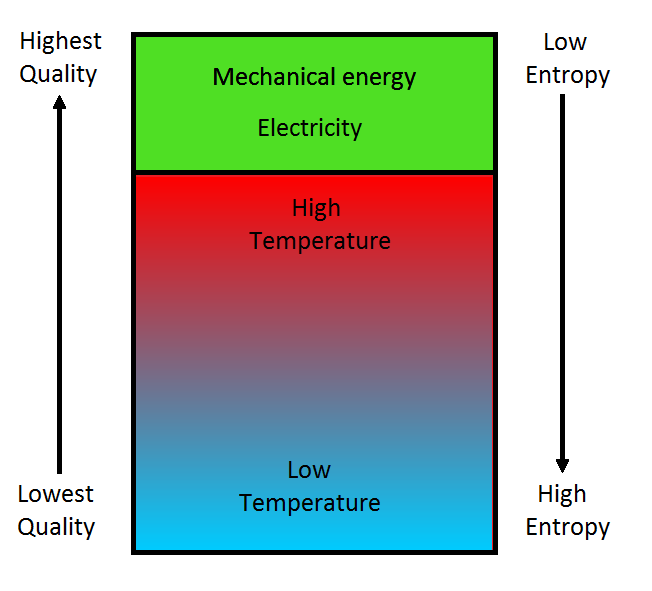

Comments
Post a Comment